 MatSE 443
MatSE 443 Introduction to the Materials Science of Polymers
Penn State University
 PC Painter and MM Coleman Fundamentals of Polymer Science (2nd Edition, CRC Press) |
Chapter 9
- Thermodynamics of Mixing:
- At the outset you must realize that the free energy of mixing, the entropy of mixing, and the enthalpy of mixing, are all differences, i.e. they describe the thermodynamic energy change between a mixed and a demixed system. Secondly, you must understand that these properties are calculated per volume.
- entropy of mixing Follow through how Flory-Huggings start from S = kB lnW and derive the entropy of mixing enumerating the arrangements of black/white "balls" on a lattice. Equations 9.8, 9.10, and 9.17 are all the same relation written for different units; can you explain the differences?
- enthalpy of mixing Understand the definition of the chi parameter (eq. 9.21). Also, based on this definition, you should appreciate how it can be applied to the FH lattice model (eq. 9.23), how it connects to solubility parameters (eq. 9.26), and the 'empirical' relation of eq. 9.27.
- *Implicit in all the above are a number (3 important ones) of assumptions the Flory-Huggins theory is based on. You should be able to pin-point them while reading through the derivation.
- Based on the above you should be able to explain to a layman: why entropy always favors mixing, what is the temperature dependance (and where it comes from), why small molecules typically mix and polymers typically don't mix
- Phase Behavior: Concepts of chemical potential, spinodal, binodal, and critical point; and how all these are connected back to the free energy of mixing.
- Chi at the critical point, incl. the empirical relation (eq. 9.42), and Theta temperature for a solution (definition and physical meaning).
- * Make sure that you go through the derivations in the text, with the emphasis inidentifying physical meanings and assumptions.
- * Study question 1a and 1b (page 335) and 7.
- **Study question 3.
The interactive module below is an Adobe Flash object (whose native support is deprecated); you will need a Flash Emulator extension for your browser: there are several available for free in your browser's extension/web store (we recommend the "Ruffle Flash Emulator" extension, also "Flash Player Emulator 2024", "Flash works Again" and "Flash Player Enabler" seem to be as good for this course's interactive modules).
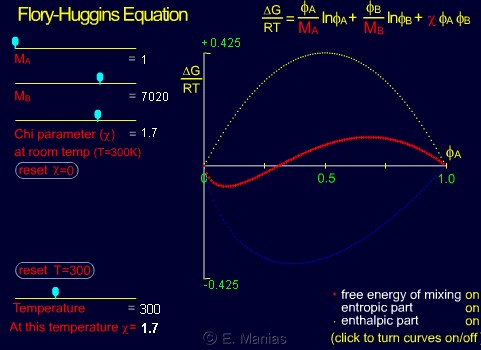
- Start by choosing MA=MB=1 (mixture of two 'monomers'), and span the whole range of available chi values. Notice how entropy remains the same (the blue curve only seems to move because the y-axis changes), and how the enthalphy changes. Can you relate these two behaviors back to the FH equation?
- Set the chi to a small positive value (0.3-0.5), and increase the MA. What do you notice for the enthalpy/entropy, as far as their dependence on MA is concerned? Can you explain this by pointing to the correct term in the FH equation?
- Do the same for any other value of chi (positive or negative). Do the MA dependances change?
- Can you calculate why such a sharp change when changing from MA=1 to a slightly higher number, and the insencitivity to MA between values of 60 to 10,000?
- What happens if both MA and MB are larger than 1? Comment on both the entropy and the enthalpy contributions to the FH free energy of mixing.
