 MatSE 443
MatSE 443 Introduction to the Materials Science of Polymers
Penn State University
 PC Painter and MM Coleman Fundamentals of Polymer Science (2nd Edition, CRC Press) |
Chapter 8
- Thermodynamics Review:
- phase transitions and how to distinguish between first-order transitions and other transitions (e.g. second-order transitions);
- 'molecular' definition of entropy (S = kB lnW ) and its relation to the number of system configurations/arrangements (W)
- thermodynamic definitions of: free energy, enthalpy, heat and work, heat capacity, compressibility
- Energetics of Crystallization: how does the free energy gain upon crystallization depend on the size of the lamella (crystal thickness or fold period, long period); how is the melting point defined through the free energy considerations (eq. 8.39)
- Kinetics of Crystallization: what is primary and secondary nucleation, what is primary and secondary crystallization; know how to qualitatively explain when you have more and smaller spherulites, rather than fewer and larger spherullites, depending on the crystallization temperature (i.e. consequences of fig. 8.6)
- Melting Temperature (Tm): qualitative understanding of DSC; good understanding of how and why Tm is affected by:
- Chemical Structure: chain/backbone flexibility, intermolecular interactions, and side group size
- Polymer molecular weight, diluents (plasticizers), and comonomers
- Glass Transition Temperature (Tg): qualitative understanding of the nature of a glass transition ('second-order' like character, structural changes, dynamical changes); concept of free volume; WLF equation and physical meaning; good understanding of how and why Tg is affected by:
- Chemical Structure: chain/backbone flexibility, intermolecular interactions, and side group size
- Polymer molecular weight
- Diluents (plasticizers) and comonomers, incl. equation to predict the Tg of the 'mixture' (Fox equation, see also the interactive module below)
- Cross-linking and Crystallization
- Important: The same polymer property (e.g. backbone flexibility) may affect the Tm and Tg in a similar manner, but this is due to completely different reasons (free energy of crystal vs. free volume in the system). It is important that you understand and can rationalize why and how!
- * Make sure that you go through the examples in the text, where different polymers are compared for their Tm and Tg, and understand why these differences are there (free energy of crystal vs. free volume)
- * Study question 3 (page 303)
The interactive module below is an Adobe Flash object (whose native support is deprecated); you will need a Flash Emulator extension for your browser: there are several available for free in your browser's extension/web store (we recommend the "Ruffle Flash Emulator" extension, also "Flash Player Emulator 2024", "Flash works Again" and "Flash Player Enabler" seem to be as good for this course's interactive modules).
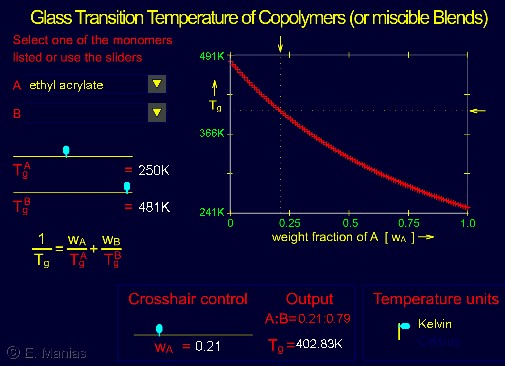
- Start by choosing the ethylene (=A) / propylene (=B) pair. Notice how Tg depends on the weight fraction of ethylene. If you were a car manufacturer and had the ability to get copolymers or blends of polyethylene and polypropylene, what type of ethylene-fractions would you use for the blend suitable for the car bumper, for the car seat, and for the air bags. (You need to take into account that both polyethylene and poly propylene crystallize and their respective Tm is 410K and 443K, you may assume it's the same for this question).
- Choose a Tg for A ca.50K, and a Tg of B ca.300K. Is the dependence of the mixture Tg a linear function? If you consider that most small-molecule materials (e.g. solvents) have very low Tg, what are the implications of adding small or large quantities of a solvent to a high Tg polymer
